- Received On: 2021-09-03|
- Accepted On: 2021-10-19|
- Published On: 2021-11-11
| Article Metadata | |||
|---|---|---|---|
| 1 | Submitted Manuscript | PPD/MIN/219/2 | |
| 2 | Cover Letter to Editor | PPD/CLE/219/2 | |
| 3 | Copyright Transfer Letter | PPD/CTL/219/2 | |
| 4 | Authors’ Consent Letter | PPD/ACL/219/2 | |
| 5 | Initial Editorial Screening Report | PPD/IESR/219/2 | |
| 6 | Review Agreement Letter (Reviewer 1) | PPD/RAL/2192/R1 | |
| 7 | Review Agreement Letter (Reviewer 2) | PPD/RAL/2192/R2 | |
| 8 | Manuscript Review Report (Round 1, Reviewer 1) | PPD/MRR/2192/R1.1 | |
| 9 | Manuscript Review Report (Round 1, Reviewer 2) | PPD/MRR/2192/R1.2 | |
| 10 | Revised Manuscript | PPD/MIN/2192R | |
| 11 | Review Response Letter (Round 1) | PPD/RRL/2192/R1 | |
| 12 | Manuscript Review Report (Round 2, Reviewer 1) | PPD/MRR/2192/R2.1 | |
| 13 | Manuscript Review Report (Round 2, Reviewer 2) | PPD/MRR/2192/R2.2 | |
| 14 | Final Editorial Screening Report | PPD/FESR/2192 | |
| 15 | Letter of Acceptance and Acknowledgement | PPD/LAA/2192 | |
| 16 | Accepted Manuscript | PPD/MIN/2192A | |
| 17 | Galley Proof Manuscript | PPD/MIN/GPM/2192 | |
| Request Access | |||
| Supplementary Data | ||
|---|---|---|
| The datasets used and/or analyzed during the study are available from the corresponding author on reasonable request. | ||
| Datasets | Not Provided | Request Availability |
Abstract
Background: Antibacterial efficacy of neem oil has been well documented in literatures for their traditional and scientific basis of use. Though plenty of research have already been conducted, very few have been focused to revive weak antibiotic standards against resistant bacteria with its combination. Thus, the present study was designed to rejuvenate penicillin with combined use of neem oil.
Methods: Four gram-positive and two gram-negative resistant bacteria were selected for the study. Antibacterial efficacy was analyzed by disc diffusion method and microdilution method where zones of inhibition were measured and percentage of inhibition were calculated.
Results: Neem oil demonstrated firm antibacterial efficacy against all bacteria in its individual application. Moreover, its combination with penicillin boosted the efficacy with synergism against S. aureus, M. luteus, E. coli and P. aeruginosa, however, on the contrary, decreased the efficacy against S. epidermis and B, megaterium.
Conclusion: The study concludes that neem oil possesses higher synergistic potential which can facilitate the efficacy of penicillin against resistant bacteria. However, further investigation is required to understand the underlying mechanism of inhibition. Significance Statement: The study obtained three new findings; first, the broad-spectrum synergistic effect of neem oil observed when combined with penicillin; provided a clue for revival of penicillin; second, the synergism against gram-negative bacteria was greater than that against gram-positive bacteria; third, unorthodoxly the combination also found to reduce the antibacterial efficacy than that of neem oil’s solo application against two gram-positive bacteria.
Introduction
Medical science and health care management is gaining new heights every single day, however, resistance to antimicrobials, which started more than 50 years ago [1], remains a worldwide public health threat that continues to create new records on mortality, morbidity rate, and increasing cost of the health care system every year. [2,3]. Antibacterial resistance has become the major global health setback regarding the treatment of infectious disease, with developing countries being the primary sufferer due to scarcity of surveillance and clinical misuse of antibiotics. [4]. Consequently, the once discovered antibiotics, are no longer in effect, and such for the case of the first discovered antibiotic penicillin, introduced as a wonder-drug. It has become resistant to almost all the bacterial strain, whereas the infectious pathogens are rising and evolving day by day. Both gram-positive and gram-negative pathogens have gained the capability to undermine one or more antimicrobial agents and led these to deploy effectiveness with extended use [3]. Moreover, the newly developed antibiotics are in shortage to give activity against the all types of infectious disease. Whereas, the availability of new novel antibacterial agent for a developing country is becoming non-viable due to economical constraint, the waiting period for new drug development fails to serve the imminent need. On the contrary, natural adjuvants are easily available hence, effective for antibacterial treatments [5]. Neem oil is one of them.
Neem tree (Azadirachta indica A. Juss), belonging to the Meliaceae family, is among one of the fundamental sources of traditional medicine in the Indian subcontinental for millennia [6,7]. It is also found in many tropical and semitropical areas around the world including Bangladesh, India, Pakistan, Indonesia, Australia, Sudan and Thailand [6]. Different parts of the neem plant containing various active constituent have been reported to show activity against different diseases [7,8]. Among different parts of the plant, the neem oil is principally extracted from the seeds [9]. The color of the neem oil is generally light to dark brown and it has an unpleasant taste and offensive odor like garlic and peanut combination [9]. Apart from its traditional use as skin protectant, anti-lice and anti-dandruff [10,11], it possesses a broad spectrum of biological activity including anti-parasitic, antimicrobial, antipyretic, anti-inflammatory, immunostimulant, antiulcer, and antifungal [7,12]. Literatures confirmed antibacterial efficacy of neem oil against S. aureus, S. typhi, K. pneumoniae, E. coli, P. aeruginosa, S. mutans, E. faecalis, L. acidophilus, H. pylori and many more pathogenic bacteria [13-16]. Moreover, the oil is also effective against viruses (HIV and Polio) and malaria parasites (P. falciparum) [17]. Despite having wide range of antimicrobial activities, most study focused on evaluating the individual potential of the oil against resistant bacteria. Only very few have been found focused so far on increasing the efficacy of weak antibiotics through combination. Thus, the present study aimed to assess the potential of neem seed oil in rejuvenating the efficacy of penicillin.
Methods
Sample Collection
Ripened neem fruits (about 500 gm) were collected from a neem tree from the location of Keraniganj Upazila (23°41′0″N, 90°20′0″E) of Dhaka District of Bangladesh in October 2020. Seeds were removed from the fruits and allowed for air dry under shade.
Extraction and Isolation
The extraction of the neem seeds followed the industrial method, steam distillation with petroleum ether, consisting of boiling point about 60–80°C [18]. Firstly, the seeds were washed thoroughly for cleaning the adhered heavy metal, dust, and other exogenous materials. After sun drying, the kernels were separated from the seeds by removing the neem husk. Then, the kernels were crushed in the mortar pestle for the final extraction where the crushed kernels (about 50 gm) were dissolved in the 50ml pet ether solvent. After that, through ordinary distillation at 70°C, oil was removed from the filtrate. Subsequently, the trace amount of solvent remaining in the oil is removed by putting the oil in a round bottom flask and placed in a water bath for 20h at 60–70°C in a rotary vacuum evaporator (Biobase RE-2010, China). The final product was 1.2 ml (100%) seed oil without any trace amount of solvent, impurities, or moisture content.
Collection of Bacterial Strains
Among six bacterial strains, four gram-positive bacteria - Staphylococcus aureus (ATCC 6538), Staphylococcus epidermidis (ATCC 12228), Micrococcus luteus (ATCC 4698), Bacillus megaterium (ATCC 14581) isolated from cough, nasal mucosa, urine and stool culture respectively; and two gram-negative bacteria - Pseudomonas aeruginosa (ATCC 27853), Escherichia coli (ATCC 25922) isolated from urine and stool samples respectively, were collected from the Center for Medical Biotechnology, Institute of Public Health, Bangladesh.
Preparation of Inoculums
Bacterial strains were sub-cultured overnight at 37°C ± 1°C in Mueller–Hilton agar (MHA) plates and in Nutrient Broth tubes diluted to achieve viable cell count of 107 CFU per ml count.
Antimicrobial Susceptibility test
The determination of antibiotic susceptibility was followed using the disc diffusion method [19]. Separate sterile cotton swab was used for each bacterial strain to streak on a 90mm prepared MHA plate. Each plate was marked into four equal zones at the outer bottom. The prepared paper disc (5mm in diameter) used for the antimicrobial test were sterilized in the autoclave at 121°C for 20 minutes and dried before soaking with the agent. The phenoxy-methyl penicillin [Square Pharmaceuticals LTD.] (10µl disc-1 of 10mg.ml-1 w/v on sterile water), neem oil (10µl disc-1) and neem oil-penicillin combination (10µl of each disc-1) were applied and placed on the agar plate. Sterile water, 10µl disc-1 was applied on the media plate as a negative control. After incorporation of all the samples, the media plates were held to the incubation chamber at 37°C ± 1°C for 24 hours and the diameters (mm) of zones of inhibition were measured. The same procedure was repeated in triplicates for each microorganism to get the best possible results and clear any uncertainty regarding the results.
Inhibition of Bacterial Growth
The microdilution techniques, as described by Patton et al., were used for the determination of the percentage growth inhibition with slight modification [20]. A microplate with 96-well was used for two-fold serial dilution of stock neem oil (100%) to generate the concentration of 50%, 25%,12.5%, 6.25% and 3.125% (v/v) using Nutrient broth (NB) medium. The diluted concentrations were further separately applied in combination with phenoxymethylpenicillin. Each well contained 155 μl of NB, 15 μl of bacterial suspension, and 15 μl of Neem oil whereas the well for the standard contained phenoxymethylpenicillin 15 μl (1µg/µl) in place of neem oil. For combination well, the penicillin (15 µl) and neem oil (15 µl) were added with a bacterial suspension of 15µl. Further, NB was added to each well to make the final volume of 250 µl. The negative control wells did not contain any antibacterial agent or antibiotics. The initial absorbance (T0) of the microwells was taken through Biobase-EL10A microplate reader (China). Then, the plates were allowed for incubation at 37°C ± 1°C for 24 hours and after the incubation, the absorbance (T24) was again taken. The difference between the (T24 and T0) were used with below formula for the calculation of the percentage of inhibition.
Percentage inhibition = 1 − (OD test/OD control) × 100
Where, OD = Optical Density.
Statistical analysis
The statistical analysis of the obtained data was represented by one-way analysis of variance (ANOVA) and p< 0.05 has been considered as statistically significant. The zone of inhibition has been analyzed in the mean ± standard deviation manner.
Results
From the measurements of zones of inhibition, it was observed that penicillin produced negligible zones against the resistant bacteria (Figure 1). However, neem oil demonstrated noticeable efficacy in producing non-bacterial zones against all specimens. In addition, neem oil’s combination with penicillin exhibited synergistic effect against all bacteria except S. epidermis and B. megaterium. Interesting fact observed in this test was that the gram-negative bacteria showed more susceptibility towards the oil-drug combination than gram-negative bacteria. Maximum zone diameter was measured against S. aureus (32.0 mm) among gram positive and E. coli (36.5 mm) among gram-negative specimens.
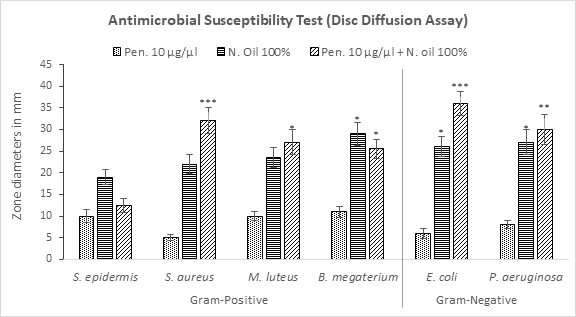
Figure 1: Measurement of zones of bacterial inhibition by penicillin, neem oil and neem oil-penicillin combinations, assayed in disc diffusion method. PEN = Penicillin (10μg/μl); N. oil = Neem oil. Data represents diameter (mm) of zone of inhibition expressed as mean ± standard deviation, (n = 3); *p < 0.05, **p < 0.01, ***p < 0.001; Dunnett t-test (two sided) treated one group as control (no antibacterial agent) and compared all other groups against it.
Neem oil established a dose dependent gradient relationship in regards to percentage of growth inhibition in gram positive bacteria such as in case of S. epidermis, exhibiting higher efficacy with higher strength (Figure 2a). This dose dependent pattern was found also true for its combination with penicillin (1µg/µl) from NO 100% (31.8%) to NO 12.5% (2.4%) after which a reversal phenomenon was observed with next two dilutions of neem oil - 6.25% (6.8%) and 3.125% (11.7%) which were associated with synergistic effects. Noticeably, all other higher conjugations caused in lower efficacies than that of their individual use. Penicillin resulted in a very negligible growth inhibition (2.8%) which was even less than that of NO 6.25%.
Figure 2b also exhibited similar pattern by neem oil against S. aureus though it demonstrated a mild inhibition in comparison till NO 6.25% (9.4%) strength afterwards, an increase in percentage inhibition was observed at NO 3.125% (11.1%). When applied together with penicillin, a remarkable increase in efficacy was observed at NO 100% (from 21.2% to 61.2%) and 50% (from 17.0% to 60.2%) indicating synergistic effects followed by a drastic fall in next three dilutions up to NO 6.25% (13.0%). A sharp increase was observed with the combination (19.2%) at NO 3.125% as like its individual application. Penicillin could not show significant inhibition of S. aureus growth and all the strength of neem oil individually or in combination with penicillin exceeded penicillin at its individual application in efficacy.
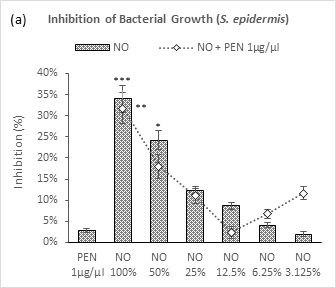
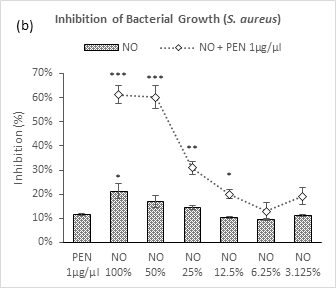
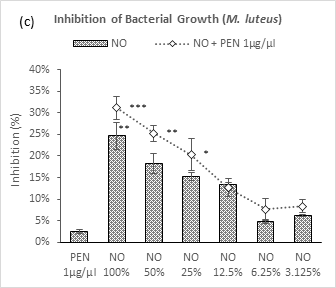
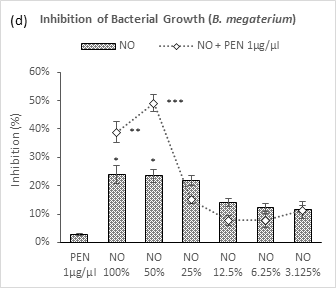
Figure 2 (a-d): Expressions of percentage of inhibition by penicillin, neem oil and neem oil-penicillin combinations against gram-positive bacteria, assayed in microdilution method. PEN = Penicillin (1μg/μl); NO = Neem oil. Graphs represent dose response curve per μg/ml concentrations, data expressed percentage of inhibition as mean ± standard deviation, (n = 3); *p < 0.05, **p < 0.01, ***p < 0.001; Dunnett t-test (two sided) treated one group as control (no antibacterial agent) and compared all other groups against it.
A gradual decrease in efficacy was observed against M. luteus with number of dilutions of neem oil and neem oil – penicillin combination except for NO 3.125% (Figure 2c). A slight increase in percentage from NO 6.25% to NO 3.125% of neem oil could attribute to experimental error. All strengths with penicillin showed synergism except by NO 12.5% which did not contribute to this pattern. Penicillin failed to produce noteworthy efficacy and all applications demonstrated better efficacy in its comparison.
In case of B. megaterium (Figure 2d), highest synergistic inhibition was observed with penicillin by NO 50% (49.1%), though NO 100% started with lower efficacy in comparison. All further dilutions applied with penicillin resulted in lower inhibition than that of their individual uses. There was no significant dose gradient relationship observed in application of neem oil alone. The first three doses of NO were found with moderate inhibition. Penicillin remained inactive as such as the cases of other gram-positive bacteria.
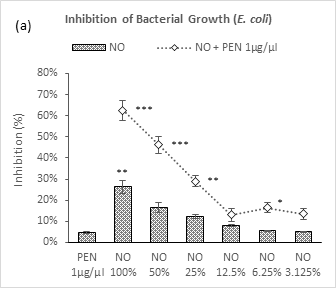
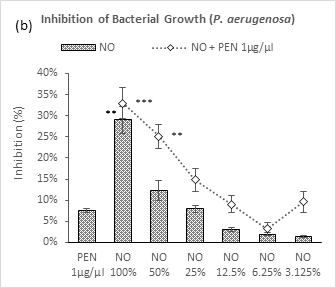
Figure 3 (a-b): Expressions of percentage of inhibition by penicillin, neem oil and neem oil-penicillin combinations against gram-negative bacteria, assayed in microdilution method. PEN = Penicillin (1μg/μl); NO = Neem oil. Graphs represent dose response curve per μg/ml concentrations, data expressed percentage of inhibition as mean ± standard deviation, (n = 3); *p < 0.05, **p < 0.01, ***p < 0.001; Dunnett t-test (two sided) treated one group as control (no antibacterial agent) and compared all other groups against it.
As like the case of gram-positive bacteria, neem oil in its individual application demonstrated a dose dependent relationship against both the gram-negative bacteria – P. aeruginosa and E. coli (Figure 3a-3b). Maximum inhibition was observed by NO 100% (29% and 26.4%) against these two bacteria respectively. Whereas, against P. aeruginosa, a moderate synergistic potential was observed by the combination with penicillin which lasted in decrement till 6.25% followed by a sharp peak at 3.125%; against E. coli, the combination demonstrated a linear fall in inhibition with dilutions till 12.5% followed by two arbitrary increases by the next two doses. Though, penicillin exhibited a very mild inhibition potential against P. aeruginosa (7.6%), showed negligible potential against E. coli (4.7%).
Discussion
In the history of antibiotics, penicillin is considered the most valuable and oldest available antibiotic that is still in use in primary care. Among the earliest discovered antibiotics, penicillin used to have broad-spectrum activity against almost all types of bacterial strains including aerobic, gram-positive, and any bacteria devoid of beta-lactamase enzyme [21,22]. As, penicillin belongs to the beta-lactam antibiotic class; the fundamental mechanism of penicillin is bactericidal because of its blocking potential of bacterial cell wall synthesis [23]. The pathway is through interruption of the transpeptidation process by which individual peptidoglycan component of the bacterial cell wall link with each other [24]. The phenomenon eventually kills the susceptible bacteria through exposure to outside water and molecular pressure [25]. However, the increased use of penicillin since its discovery arises the issue of resistance against almost all bacteria.
Bacterial resistance against the antimicrobial agents is gained by either of the four different ways, such as, natural resistance, acquired resistance, cross-resistance and multi-drug resistance and pan-resistance [26]. One way is that the bacterial outer membrane obstructs the penetration of phenoxy methyl penicillin whereas the other resistance reasons are acquired, such as, secretion of beta-lactamase enzyme or penicillinase, efflux, and mutation [27].
Eventually, the beta-lactam ring, the pharmacophore of the phenoxymethylpenicillin is destructed by the beta-lactamase, and/or, penicillin affinity for the target site is altered. [28]. Among different bacterial resistance mechanism, gram-positive bacteria gain resistance through two major strategies: enzymatic degradation of antibiotic by the production of β-lactamases, or by decreasing the affinity and susceptibility of their target site, the penicillin-binding protein (PBP), by either acquisition of exogenous DNA or by changes in the native PBP genes [29,30]. In accordance with gram-positive features, the gram-negative bacteria obtain resistance against the penicillin through producing extended-spectrum β-lactamase where it causes the destruction of β -lactam by catalyzing the hydrolysis of the antibiotics along with alteration of outer membrane porin channels [31,32].
As a potential folklore medicine, neem oil has been used in a wide range of disciplinary of medicine for example Ayurveda, Homeopathic and Unani and give protection against a wide range of micro-organisms [33-35]. Approximately, 135 chemical components have been isolated from different parts of the neem [36]. Neem oil also constitutes several types of phytochemicals including azadirachtin, gedunin, isomargolonone, margolone, margolonone, nimbidin, nimbin, nimbolide and salannin [37,38]. The nimbolide component of the oil extracts play a key role in antibacterial properties. Other than nimbolide, the mahmoodin, nimbidin, and crude bitter extracts of the oil play a key role in antifungal, antimalarial and antiviral properties [7, 35,39,].
In this study, neem oil was found to inhibit both gram-positive and gram-negative bacteria better than that exhibited by penicillin. Moreover, the application of neem oil together with penicillin resulted in synergistic efficacy against all tested bacteria except for B. megaterium and S. epidermis. Though, in case of B. megaterium, synergism was observed with higher doses (100% and 50%) of neem oil, interestingly a complete subtraction in efficacy was observed against S. epidermis except for last two doses (6.25% and 3.125%). This arises a new focus for further investigation. From the data of both disc diffusion and microdilution test, it can be concluded that the oil-drug combination significantly produced antibacterial efficacy against S. aureus and M. luteus but failed to failed against S. epidermis and B. megaterium among the gram-positive bacteria. On the contrary the combination proved to be highly effective against both E. coli and P. aeruginosa representing more susceptibility of gram-negative bacteria towards it.
The oil has been previously reported to exert bactericidal effect by the inhibition of cell membrane synthesis [40,41]. Although the underlying mechanism is still unknown, till now the established mechanism of nimbolide is disintegrating the bacterial cell envelope. As a consequence, severe bacterial membrane perturbation results by membrane damage, bursting of cells and lastly cell lysis [42-44]. Additionally, the new limonoid constituent, mahmoodin gives a broad spectrum of activity against both gram-positive and gram-negative bacteria. Alongside, the tetranonriterpenoids, nimbidin showed significant antibacterial activity [12,41,45,].
Conclusion
The folk use of neem oil for antibacterial properties is well established. The current study provided a scientific basis of its antibacterial use. Moreover, its combination with penicillin facilitates for a synergistic potential against both gram-positive and gram-negative bacteria. Further investigation is recommended to find out involved mechanism of inhibition.
Abbreviations
PEN: Penicillin; NO: Neem Oil; MHA: Muller Hinton Agar; NB: Nutrient Broth; ATCC: American Type Culture Collection.
Ethics Approval and Consent to Participate
The work was exempt for ethical approval. All experiments were performed in accordance with Clinical and Laboratory Standards Institute (CLSI)’s standards for microbial testing - M02: Performance Standards for Antimicrobial Disk Susceptibility Tests, 13th Edition; M100: Performance Standards for Antimicrobial Susceptibility Testing, 31st Edition; M26: Methods for Determining Bactericidal Activity of Antimicrobial Agents, 1st Edition; M29: Protection of Laboratory Workers From Occupationally Acquired Infections, 4th Edition, adopted in the Institutional Standard Microbial Testing Procedures (Ref. no. IPSDRLAB/ ISMTP/01/19). The experimental design was authorized by the Institutional Ethical Committee Clearance (Ref. No. IPSDRLAB/IECC/04/20) of the Institute for Pharmaceutical Skill Development and Research, Bangladesh (Project approved on 17/02/2019).
Acknowledgments
The present study was supported and carried out in the Pharmacology lab of Institute for Pharmaceutical Skill Development and Research, Bangladesh. Authors are also grateful to the Center for Medical Biotechnology under MIS, DGHS, for providing the bacterial strains used in this study.
Authors’ Contributions
This work was carried out in collaboration between all authors. Authors MNI and MMB designed, coordinated and supervised the project. AHO and FA performed the experiments. AHO prepared the manuscript. FA critically revised the manuscript. MMB performed the statistical analysis, prepared the graphical presentations.
Consent for Publication
Not applicable.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors. The study was carried out with individual funding of all authors.
Availability of Data and Materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Competing Interests
All authors agreed on the article before submission and had no conflict of interests.
References
- Levy SB. Antibiotic and antiseptic resistance: impact on public health. The Pediatric infectious disease journal. 2000 Oct 1;19(10):S120-2. PubMed Google Scholar Link
- Shlaes DM, Gerding DN, John Jr JF, et al. Society for Healthcare Epidemiology of America and Infectious Diseases Society of America Joint Committee on the Prevention of Antimicrobial Resistance: guidelines for the prevention of antimicrobial resistance in hospitals. Infection Control & Hospital Epidemiology. 1997 Sep 1;25(3):584-99. DOI PubMed Google Scholar Link
- Ang JY, Ezike E, Asmar BI. Antibacterial resistance. The Indian Journal of Pediatrics. 2004 Mar 1;71(3):229-39. DOI PubMed Google Scholar Link
- Chokshi A, Sifri Z, Cennimo D, Horng H. Global contributors to antibiotic resistance. Journal of global infectious diseases. 2019 Jan;11(1):36-42. PubMed PMC Google Scholar Link
- Douafer H, Andrieu V, Phanstiel IV O, Brunel JM. Antibiotic adjuvants: make antibiotics great again!. J Med Chem. 2019 May 7;62(19):8665-81. DOI PubMed Google Scholar Link
- Srirangarayan RS, Ramasamy M, Paulin Thangam R. Hidden Elixir in Indian Sub Continent–Azadirachta Indica Linn. Social Science Research Network; 2020. DOI Google Scholar Link
- Biswas K, Chattopadhyay I, Banerjee RK, Bandyopadhyay U. Biological activities and medicinal properties of neem (Azadirachta indica). Current Science. 2002;82(11):1336-1345. Google Scholar Link
- Sharma Y. Comparative study of different parts of Azadirachta indica (neem) plant on the basis of antibacterial activity, phytochemical screening and its effect on rat pc-12 (pheochromocytoma) cell line. International Journal of Biotechnology, Humanities, Sports and Allied Fields. 2014;2:144-154. Google Scholar Link
- Tesfaye B, Tefera T. Extraction of essential oil from neem seed by using soxhlet extraction methods. International Journal of Advanced Engineering, Management and Science. 2017;3(6):239870. Google Scholar Link
- Tomar A, Verma G, Phogat S, Singh M. Neem in Health and Cosmetics. Neem: A Treatise, IK International Publishing House Pvt Ltd., New Delhi. 2009:461-85. Google Scholar Link
- Abdel-Ghaffar F, Semmler M. Efficacy of neem seed extract shampoo on head lice of naturally infected humans in Egypt. Parasitol Res. 2007;100(2):329-332. DOI PubMed Google Scholar Link
- Kaur G, Sarwar Alam M, Athar M. Nimbidin suppresses functions of macrophages and neutrophils: relevance to its antiinflammatory mechanisms. Phytotherapy Research. 2004;18(5): 419-24. DOI PubMed Google Scholar Link
- Jahan T, Begum ZA, Sultana S. Effect of neem oil on some pathogenic bacteria. Bangladesh Journal of Pharmacology. 2007 Jun;2(2):71-72. DOI Google Scholar Link
- Menon GR, Vishnupriya V, Gayathri R, Geetha RV. Anti-bacterial activity of neem oil on oral pathogens – an in vitro study. Int. J. Pharm. Sci. Rev. Res. 2016;39(1): 219-220. Google Scholar Link
- Sultana S, Shova NA, Ahmed A, Hossain MM. Comparative study on the antibacterial activities of neem oil, mustard oil and black seed oil against staphylococcus aureus, klebsiella pneumoniae, bacillus cereus, salmonella typhi and pseudomonas aeruginosa. European Journal of Scientific Research. 2019 Sep;154(1):58-67. Google Scholar Link
- Blum FC, Singh J, Merrell DS. In vitro activity of neem (Azadirachta indica) oil extract against Helicobacter pylori. Journal of ethnopharmacology. 2019 Mar 25; 232:236-43. DOI PubMed Google Scholar Link
- Chauhan M, Mani P, Sharma TK. The Antibacterial Activities of Neem [Azadirachta Indicia] Seed Oil, A Review. IOSR Journal of Applied Chemistry. 2018 May;11(5):58-63. Google Scholar Link
- Kumar D, Rahal A, Malik JK. Chapter 43 - Neem Extract. In: Gupta RC, ed. Nutraceuticals. Academic Press; 2016:585-597. DOI Google Scholar Link
- Balouiri M, Sadiki M, Ibnsouda SK Methods for in vitro evaluating antimicrobial activity: A review. Journal of pharmaceutical analysis. 2016 Apr 1;6(2):71-9. doi:10.1016/j.jpha.2015.11.005 DOI PubMed PMC Google Scholar Link
- Patton T, Barett J, Brennan J, Moran N. Use of a spectrophotometric bioassay for determination of microbial sensitivity to manuka honey. J Microbiol Methods. 2005; 64:84-95. DOI PubMed Google Scholar Link
- Miller EL. The penicillins: a review and update. Journal of midwifery & women's health. 2002 Nov 1;47(6):426-34. DOI PubMed Google Scholar Link
- Hossain R, Rahman MS, Rayhan MA, Nawrin K, Billah MM, Habib MR. Antibacterial efficacy of black seed honey in combination with penicillin and amoxiclav against gram-positive bacteria. International Journal of Scientific Reports. 2020 Feb 1; 6(2):61-66. DOI Google Scholar Link
- Donowitz GR, Mandell GL. Beta-lactam antibiotics. New England Journal of Medicine. 1988 Feb 18;318(7):419-26. DOI Google Scholar Link
- Neu HC. Penicillins--new insights into their mechanisms of activity and clinical use. Bulletin of the New York Academy of Medicine. 1982 Nov;58(8):681. PMC Google Scholar Link
- Yocum RR, Rasmussen JR, Strominger JL. The mechanism of action of penicillin. Penicillin acylates the active site of Bacillus stearothermophilus D-alanine carboxypeptidase. Journal of Biological Chemistry. 1980 May 10;255(9):3977-86. DOI Google Scholar Link
- Tenover FC, Hughes JM. The challenges of emerging infectious diseases: development and spread of multiply-resistant bacterial pathogens. Jama. 1996 Jan 24;275(4):300-4. DOI PubMed Google Scholar Link
- Lee NL, Yuen KY, Kumana CR. β-Lactam antibiotic and β-lactamase inhibitor combinations. Jama. 2001 Jan 24;285(4):386-8. DOI PubMed Google Scholar Link
- Khan M, Islam Z, Chowdhury AS, Yousuf SA, Amin MR, Rayhan MA. Wild honey facilitates antibacterial efficacy of penicillin and amoxicillin- clavulanic acid. Journal of Apitherapy. 2020 Apr 6;7(2):22-30. DOI Google Scholar Link
- Munita JM, Bayer AS, Arias CA. Evolving resistance among Gram-positive pathogens. Clinical Infectious Diseases. 2015 Sep 15;61(suppl_2):S48-57. DOI PubMed PMC Google Scholar Link
- Berger-Bächi B. Resistance mechanisms of gram-positive bacteria. International Journal of Medical Microbiology. 2002 Jan 1;292(1):27-35. DOI PubMed Google Scholar Link
- Oliphant CM, Eroschenko K. Antibiotic resistance, part 2: gram-negative pathogens. The Journal for Nurse Practitioners. 2015 Jan 1;11(1):79-86. DOI Google Scholar Link
- Paterson DL, Bonomo RA. Extended-spectrum β-lactamases: a clinical update. Clinical microbiology reviews. 2005 Oct;18(4):657-86. DOI PMC Google Scholar Link
- Chopra IC, Gupta KC, Nazir BN. Preliminary study of anti-bacterial substances from Melia azidirachta. Indian J Med Res. 1952 Oct;40(4):511-5. PubMed Google Scholar Link
- Elavarasu S, Abinaya P, Elanchezhiyan S. Evaluation of anti-plaque microbial activity of Azadirachta indica (neem oil) in vitro: A pilot study. Journal of pharmacy & bioallied sciences. 2012 Aug;4(Suppl 2):S394. DOI PubMed PMC Google Scholar Link
- Upadhyay RK, Dwivedi P, Ahmad S. Screening of antibacterial activity of six plant essential oils against pathogenic bacterial strains. Asian J Med Sci. 2010 Jun 25;2(3):152-8. Google Scholar Link
- Singh UP, Maurya S, Singh DP. Phenolic acids in neem (Azadirachta indica) a major pre-existing secondary metabolite. Journal of herbal pharmacotherapy. 2005 Jan 1;5(1):35-43. DOI PubMed Google Scholar Link
- Ghosh V, Sugumar S, Mukherjee A, Chandrasekaran N. Neem (Azadirachta indica) oils. In Essential Oils in Food Preservation, Flavor and Safety 2016 Jan 1 (pp. 593-599). Academic Press. Link
- Hoque MM, Bari ML, Inatsu Y, Juneja VK, Kawamoto S. Antibacterial activity of guava (Psidium guajava L.) and neem (Azadirachta indica A. Juss.) extracts against foodborne pathogens and spoilage bacteria. Foodborne pathogens and disease. 2007 Dec 1;4(4):481-8. DOI PubMed Google Scholar Link
- Gupta S, Bhat G. Antibacterial effect of Neem oil on Methicillin resistant Staphylococcus aureus. Journal of Medicinal Plants Studies. 2016 Dec;4(1):01-3. Google Scholar Link
- Baswa M, Rath CC, Dash SK, Mishra RK. Antibacterial activity of Karanj (Pongamia pinnata) and Neem (Azadirachta indica) seed oil: a preliminary report. Microbios. 2001 Jan 1;105(412):183-9. PubMed Google Scholar Link
- Girish K, Shankara BS. Neem–a green treasure. Electronic journal of Biology. 2008;4(3):102-11. Google Scholar Link
- Sarkar P, Acharyya S, Banerjee A, Patra A, Thankamani K, Koley H, Bag PK. Intracellular, biofilm-inhibitory and membrane-damaging activities of nimbolide isolated from Azadirachta indica A. Juss (Meliaceae) against meticillin-resistant Staphylo-coccus aureus. Journal of medical microbiology. 2016 Oct 18;65(10):1205-14. DOI PubMed Google Scholar Link
- Tyagi P, Singh M, Kumari H, Kumari A, Mukhopadhyay K. Bactericidal activity of curcumin I is associated with damaging of bacterial membrane. PloS one. 2015 Mar 26;10(3):e0121313. DOI PubMed PMC Google Scholar Link
- Huang E, Yousef AE. The lipopeptide antibiotic paenibacterin binds to the bacterial outer membrane and exerts bactericidal activity through cytoplasmic membrane damage. Applied and environmental microbiology. 2014 May 1;80(9):2700-4. DOI PubMed PMC Google Scholar Link
- Siddiqui S, Faizi S, Siddiqui BS. Constituents of Azadirachta indica: isolation and structure elucidation of a new antibacterial tetranortriterpenoid, mahmoodin, and a new protolimonoid, naheedin. Journal of Natural Products. 1992 Mar;55(3):303-10. DOI PubMed Google Scholar Link
Update History
| Revision Number | Date | Details Of Changes |
|---|---|---|
| 01 | 2021-11-11 | Original Article; published at its accepted version (Reference Number: PPD/MIN/2192A) |
 Pharmacotherapy & Pharmascience Discovery
Pharmacotherapy & Pharmascience Discovery
