- Received On: 2021-06-03|
- Accepted On: 2021-06-15|
- Published On: 2021-06-16
| Article Metadata | |||
|---|---|---|---|
| 1 | Submitted Manuscript | PPD/MIN/216/2 | |
| 2 | Cover Letter to Editor | PPD/CLE/216/2 | |
| 3 | Copyright Transfer Letter | PPD/CTL/216/2 | |
| 4 | Authors’ Consent Letter | PPD/ACL/216/2 | |
| 5 | Initial Editorial Screening Report | PPD/IESR/216/2 | |
| 6 | Review Agreement Letter (Reviewer 1) | PPD/RAL/2162/R1 | |
| 7 | Review Agreement Letter (Reviewer 2) | PPD/RAL/2162/R2 | |
| 8 | Manuscript Review Report (Round 1, Reviewer 1) | PPD/MRR/2162/R1.1 | |
| 9 | Manuscript Review Report (Round 1, Reviewer 2) | PPD/MRR/2162/R1.2 | |
| 10 | Revised Manuscript | PPD/MIN/2162R | |
| 11 | Review Response Letter (Round 1) | PPD/RRL/2162/R1 | |
| 12 | Manuscript Review Report (Round 2, Reviewer 1) | PPD/MRR/2162/R2.1 | |
| 13 | Manuscript Review Report (Round 2, Reviewer 2) | PPD/MRR/2162/R2.2 | |
| 14 | Final Editorial Screening Report | PPD/FESR/2162 | |
| 15 | Letter of Acceptance and Acknowledgement | PPD/LAA/2162 | |
| 16 | Accepted Manuscript | PPD/MIN/2162A | |
| 17 | Galley Proof Manuscript | PPD/MIN/GPM/2162 | |
| Request Access | |||
| Supplementary Data | ||
|---|---|---|
| The datasets used and/or analyzed during the study are available from the corresponding author on reasonable request. | ||
| Datasets | Not Provided | Request Availability |
Abstract
Background: Honey, derived from different botanical sources, have already been reported for the scientific basis of their antibacterial efficacy, however, varied to a great extent when sourced from different geographical locations. Thus, this study was aimed to highlight the antibacterial potential of honey derived from Bangladesh, Germany and Australia as well as to assess their potency to enhance the efficacy of standard antibiotics against resistant bacteria.
Methods: In vitro susceptibility of four resistant gram-positive bacterial strains to honey samples and their combinations with standard antibiotics (penicillin and amoxiclav) were investigated. The evaluation of antibacterial activity was performed by disc diffusion method as well as microdilution method using two-fold dilutions of honey samples ranging from 12.5µg/ml to 0.78µg/ml. Zone of inhibition, percentage inhibition, MIC and MBC were observed in this regard.
Results: The results revealed that tested honey samples exhibited noticeable antibacterial activity against the bacterial strains. Maximum inhibition zone was observed by Bangladeshi honey (20.4mm) against M. luteus and highest inhibition percentage was demonstrated by German honey against B. subtilis (82%). Combinations with amoxiclav showed nearly two-fold increase in efficacy by all honeys, when treated against all species.
Conclusion: The findings from this study suggested that, in addition to having inhibitory effects, all honey also enhanced the pharmacological action of penicillin and amoxiclav and could generate a potential research focus to establish a supplement for these antibiotics.
Introduction
Antimicrobial agents play an essential role in controlling the global burden of infectious diseases (1). However, due to the lack of proper knowledge on antibiotic usage, these drugs have been misused for a long period. This eventually led to the development of resistant pathogenic microorganisms and reduction in the efficacy of antibiotics (2). Antimicrobial resistance (AMR) poses a great threat to the public health, causing over 700,000 deaths per year worldwide. AMR is not only a major concern in developing countries but also in developed countries like Europe and North America, where over 20,000 people succumb to bacterial infections every year (3). It is projected that by 2050 AMR will cost approximately 10 million lives and around US$100 trillion per year, if no alternative approach is developed by then to stop its progress (4). Therefore, the emerging problem of AMR has produced a need for novel antimicrobial agents.
The practice of using natural products to treat several human ailments has been around since the evolution of mankind. Apitherapy, use of honey bee products, is one of the oldest traditional medicines for treating infections and promoting health (5). Honey offers a wide range of biological properties including wound healing and antimicrobial activity and thus gained significant attention over the past decades (6). Therefore, many studies have been focused on the use of honey in modern medicine as a promising alternative approach to combat resistant pathogens (7).
Honey is primarily a concentrated solution of carbohydrates that contains over 200 compounds including enzymes, vitamins, proteins, lipids, amino and organic acids, minerals and phytochemicals. Variations in the chemical composition of different honey are associated with the climate, botanical/ floral source and environmental condition7. For instance, Australian Leptospermum based honey exhibits efficacy against Staphylococcus aureus, Scottish blossom honey against Acinetobactor calcoaceticus, Algerian eucalyptus honey against Clostridium perfringens, Cuban christmas vine against Bacillus subtilis and so on (8-11).
Many studies have reported that the usage of honey, in combination with commonly prescribed antibiotics resulted in additive or synergistic effects against different bacterial species. Manuka honey and rifampicin combination was tested against MRSA strains that increased the susceptibility of the bacteria to the antibiotic (12). Moreover, synergism with other bee products like propolis and antibacterial agents has also been investigated (13). In previous studies, strong synergistic activity was noticed with Bangladeshi wild honey black seed honey and lychee honey with penicillin/ amoxiclav against a wide range of bacterial strains (14-16).
This study aimed to expand the investigation of synergism of honey from different geographical area having different floral origins, to achieve a wide range of antimicrobial activity. Thus, the purpose of this study is to (i) test potentials of honeys and conventional antibiotics (penicillin and amoxiclav); and (ii) examine the potential of honey in enhancing the efficacy of the antibiotics against resistant bacteria. Such combinations could lead to the development of novel antimicrobials agents that could prevent the problem of emerging resistant bacterial strains, with a broad-spectrum coverage and improved therapeutic efficiency.
Methods
Collection and Preparation of Samples
Around 1kg of honey from each source (Bangladesh, Germany and Australia) were collected directly from bee keepers in between March-June, 2019. These honeys were strained using a 0.5mm mesh to remove any coarse particles (such as pollen, beeswax etc.). It was stored in an impermeable glass container at 25°C ± 2°C, to prevent moisture accumulation.
Qualitative Physicochemical Analysis
The qualitative analysis of the physicochemical properties of the honey was carried out using the standard procedures (17).
Collection of Bacterial Strains
Four gram-positive isolates used in this study were Staphylococcus aureus (ATCC 6538), Streptococcus pyogenes (ATCC 19615), Bacillus subtilis (ATCC 6633) and Micrococcus luteus (ATCC 4698). The clinical isolates, from urine culture, were received as a gift from the Center for Medical Biotechnology, Institute of Public Health, Bangladesh.
Agar Disc Diffusion Assay
Muller Hinton agar (MHA) was used for the inoculum of bacterial cultures (18). Each bacterial strain was suspended in 5ml of sterile saline and diluted to achieve a viable cell count of 107 CFU ml−1. This suspension was streaked over freshly prepared 90-mm MH agar medium plates. Following inoculation, prepared sterile paper discs of 5-mm (in diameter) were placed into the agar plate using sterile forceps. Subsequently, 20 μl of the natural agent (honey) was added to each disc. 10 μl of 2μg/ml of phenoxymethylpenicillin (PEN) [Sanofi Aventis (BD) Ltd.] and amoxicillin/clavulanic acid (AMX) [Sanofi Aventis (BD) Ltd.] were used as positive controls and sterile distilled water was used as a negative control. The plates were incubated overnight at 37°C ± 1°C for 24 hours. After incubation, the plates were observed for zones of inhibition and diameter of the zones were measured with a Vernier Caliper’s scale. Each assay was repeated in triplicates (7).
Determination of Percentage of Inhibition
Cell suspensions of the clinical isolates were prepared in Nutrient Broth (NB) media and their concentrations were adjusted to 107 CFU/ml. Two-fold dilutions of honey samples were prepared with NB to obtain concentrations as 12.5, 6.25, 3.125, 1.56 and 0.78 µg/ml for each honey and were tested against each type of microorganism (19). The prepared concentrations (of honey samples) were also combined with phenoxymethylpenicillin and amoxicillin/ clavulanic acid in separate wells for comparison. Each well contained 200 μl of NB, 10 μl of bacterial suspension and 10 μl of antibiotic, whereas the test/sample well contained 20 μl of honey concentrations instead of the antibiotic to generate final concentrations. The combination wells contained both antibiotic (10 μl) and honey concentration (20 μl). NB was added to make the final volume of 300 μl in each well. An initial absorbance value was recorded before incubation (T0) at 630 nm using a Biobase-EL10A microplate Reader (China) (2). The plates were then incubated for 24 h at 37 °C and absorbance was measured (T24) at the same wavelength. The experiment was performed in triplicates (19). The percentage inhibition was calculated from the difference of bacterial growth using the following formula:
Inhibition (%) = {1 − OD Test/OD Control)}× 100
Where, OD is Optical Density.
Minimum inhibitory concentration (MIC)
From the broth dilution assay mentioned above, minimum inhibitory concentration (MIC) was recorded. The minimum concentration at which no growth (turbidity) was observed was considered to be the MIC for the agent or the combination (20).
Minimum bactericidal concentration (MBC)
Like MIC, MBCs of all samples were determined from the broth dilution assay. 20 μl aliquots of the suspension from MIC wells and its successive higher concentrations were transferred to separate fresh MHA plates to determine the MBC. The agar plates were incubated at 37°C ± 1°C for 24 hours. The lowest concentration of honey that showed no visible bacterial growth on the surface of the agar was recorded as the MBC (21).
Statistical analysis
All experiments were performed in triplicate and data were expressed as the mean ± standard deviation. One Way Analysis of Variance (ANOVA) followed by Dunnett’s t-test was conducted to compare all the groups with negative control and the difference was considered significant when the p value obtained was less than 0.05.
Results
Qualitative Physicochemical Analysis
The qualitative investigation of physicochemical parameters exhibited that all three honeys were high in soluble sugar content, acidic in nature and possess low moisture as shown in Table 1.
Agar Disc Diffusion Assay
An initial screening of the zones of inhibition with agar-disc diffusion assay showed GER-H to have highest antibacterial activity against Staphylococcus aureus (12.6mm) and Bacillus subtilis (19.0mm) whereas its treatment with the standards exhibited around two-fold increase in efficacy (29.7mm and 34.6mm respectively) for amoxiclav (Figure 1). Combination with penicillin displayed less synergism in comparison. On the other hand, BD-H peaked against Streptococcus pyogenes (12.7mm) and Micrococcus luteus (20.4mm). In addition, its treatment with amoxiclav significantly elevated the growth inhibition (24.4mm and 35.8mm respectively), though with penicillin demonstrated smaller zones of inhibition against the tested bacteria. In contrast, antibacterial effects exerted by AUS-H were less potent against S. aureus (7.3mm), B. subtilis (13.1mm), S. pyogenes (6.9mm) and M. luteus (18.1mm); as demonstrated by smaller inhibition zones. Its combinations also yielded with reduced zone diameters following treatment with amoxiclav and penicillin in comparison with other two honeys. Penicillin and amoxiclav alone showed maximum efficacy against M. luteus (7.8 mm and 10.2 mm) and minimum towards S. pyogenes (7.8 mm and 10.2 mm) respectively. The standards in most cases demonstrated similar growth inhibition patterns for the different test bacteria with slight variations.
Table 1: Physicochemical properties of honey samples
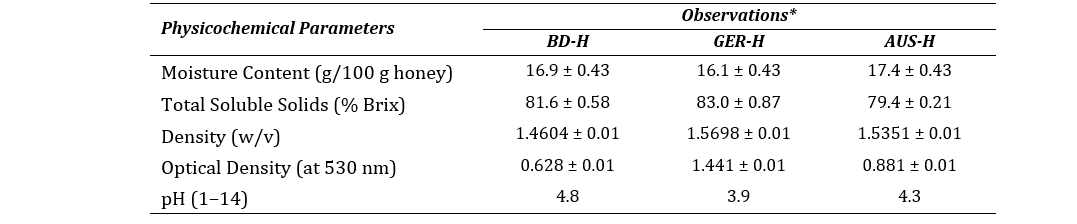
*All the methods performed in triplicate. Data represented as mean ± SEM (n=3).
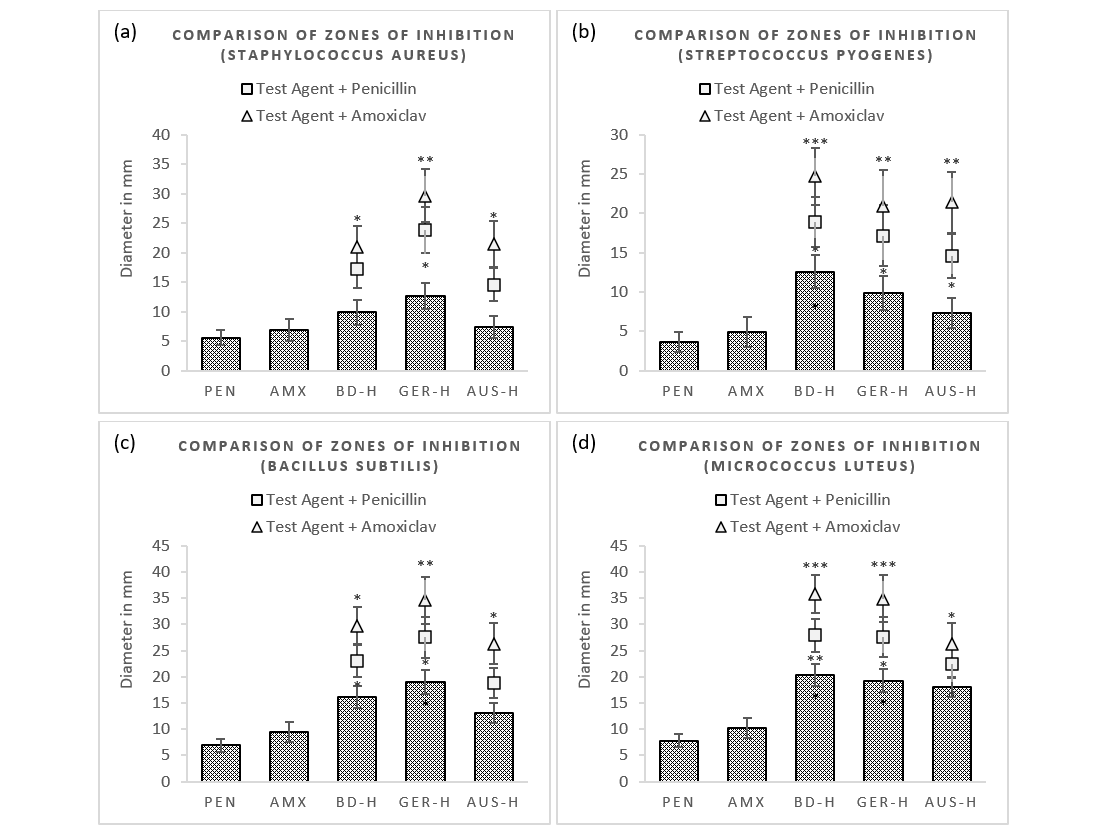
Figure 1 (a-d): Measurement of zones of inhibition of test samples and their combinations with standards in disc diffusion method.
PEN = Penicillin (2µg/ml); AMX = Amoxicillin + Clavulanic Acid (2µg/ml); BD-H = Honey sourced from Bangladesh (12.5µg/ml); GER-H = Honey sourced from Germany (12.5µg/ml); AUS-H = Honey sourced from Australia (12.5µg/ml). Data represents diameter (mm) of zone of inhibition expressed as mean ± standard deviation, (n = 3); *p < 0.05, **p < 0.01, ***p < 0.001; Dunnett t-test (two sided) treated one group as control (no antibacterial agent) and compared all other groups against it.
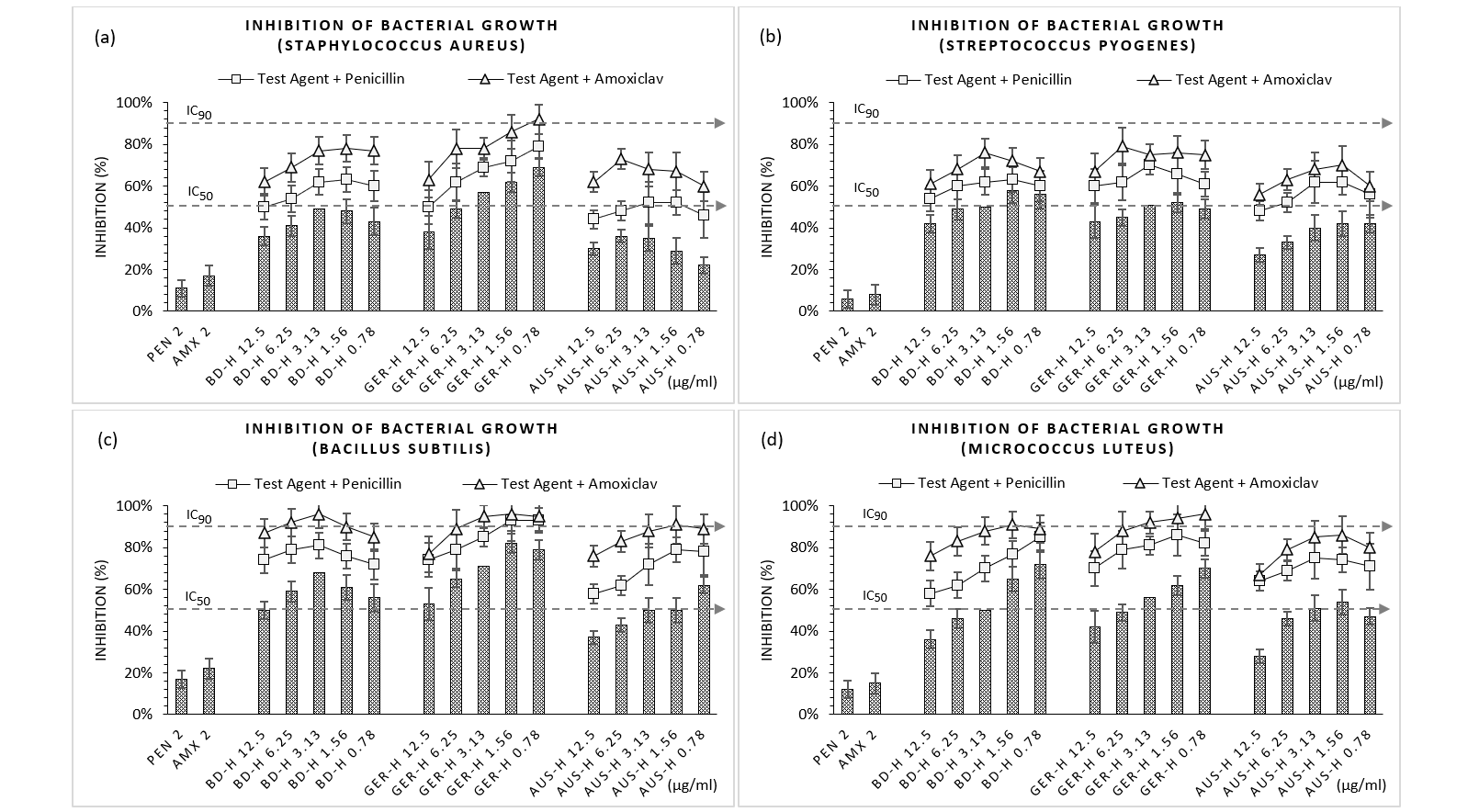
Figure 2 (a-d): Expressions of percentage of inhibition by test samples and their combinations with standards in microdilution method.
PEN = Penicillin; AMX = Amoxicillin + Clavulanic Acid; BD-H = Honey sourced from Bangladesh; GER-H = Honey sourced from Germany; AUS-H = Honey sourced from Australia. Graphs represent dose response curve per µg/ml concentrations, data expressed percentage of inhibition as mean ± standard deviation, (n = 3); *p < 0.05, **p < 0.01, ***p < 0.001; Dunnett t-test (two sided) treated one group as control (no antibacterial agent) and compared all other groups against it.
Determination of Percentage of Inhibition
Figure 2a demonstrated that alone BD-H exhibited maximum inhibition at 3.13 µg/ml followed by a fall, GER-H at 0.78 µg/ml and AUS-H at 6.25 µg/ml followed by a sharp decrease in efficacy towards S. aureus. Similar patterns were observed for B. subtilis except by AUS-H where a linear increase in inhibition was depicted (62%, 0.78 µg/ml) (Figure 2b-2d). This linearity was also found in common for S. pyogenes by AUS-H (42%, 0.78 µg/ml) and M. luteus by BD-H (70%, 0.78 µg/ml) and GER-H (68%, 0.78 µg/ml). Maximum inhibitory potential among the honeys was elucidated by GER-H 1.56 µg/ml (82%) against B. subtilis whereas lowest was illustrated by AUS-H 0.78 µg/ml (22%) towards S. aureus. Like the susceptibility tests, both the standards could not demonstrate significant growth inhibition capacity; maximum over B. subtilis (PEN 2µg/ml, 17% and AMX 2µg/ml, 22%). As observed in Figure 2 (a-d), combinations of honeys with penicillin and amoxiclav showed greater efficacy with diluted concentrations of honey contrary to the effect of GER-H with AMX against S. pyogenes where higher concentration dominated for highest inhibition (79%, 6.25 µg/ml). Uppermost inhibitions by PEN and AMX combinations were facilitated by GER-H as – S. aureus (78% and 92%), S. pyogenes (70% and 79%), B. subtilis (93% and 96%), and M. luteus (86% and 96%). While most often honeys at their individual use showed less than 50% inhibition (IC50), their combinations demonstrated up to 90% inhibition (IC90) indicating noteworthy synergism.
Table 2: Summary of Figure 2 (a-d); p value, MIC, MBC.
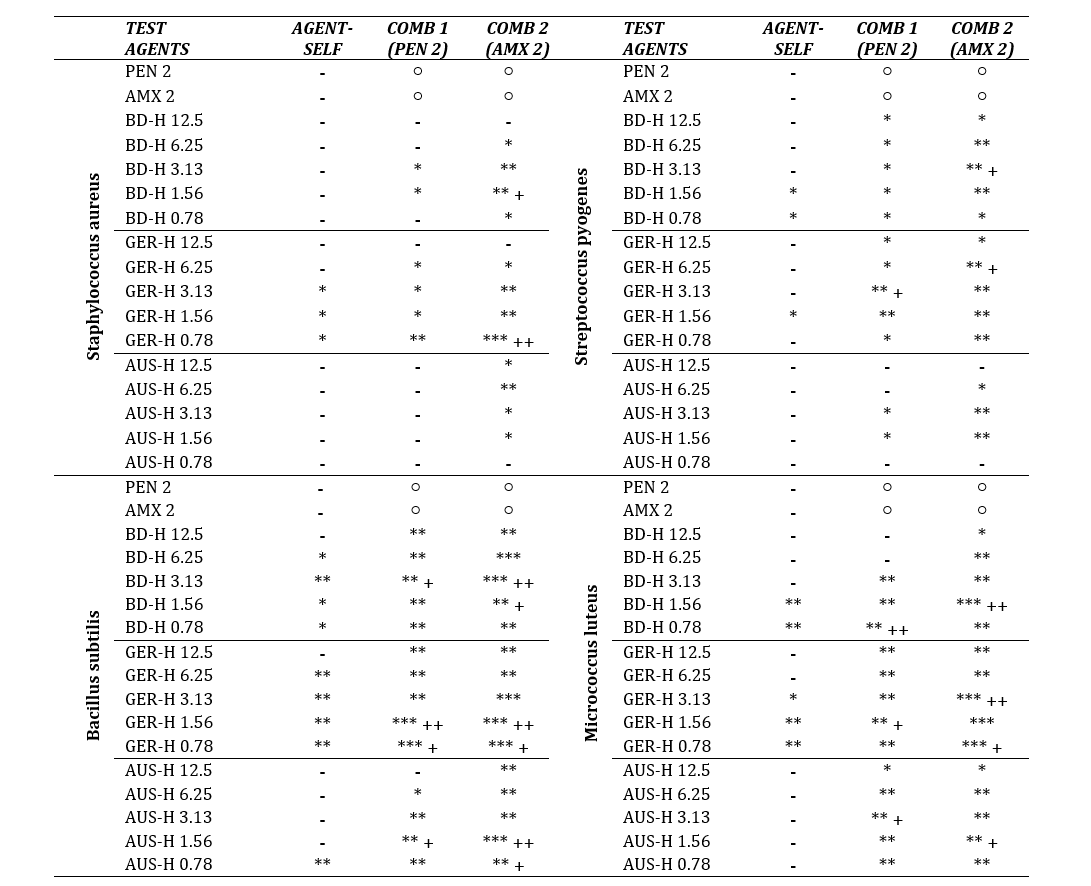
*, **, *** denotes p<0.05, p<0.01, p<0.001 respectively; + denotes MIC; ++ denotes MBC (obtained from further subculture); - denotes Not Achieved; ○ denotes Not Applicable. PEN 2 = Penicillin (2µg/ml); AMX 2 = Amoxicillin + Clavulanic Acid (2µg/ml).
Determination of MICs and MBCs
In case of Staphylococcus species (Table 2), MIC was obtained by BD-H at 1.56 µg/ml and GER-H at 0.78 µg/ml with AMX; MBC obtained for GER-H at the same concentration. S. pyogenes showed sensitivity towards both BD-H+AMX and GER-H+PEN combinations at 3.13 µg/ml and GER-H+AMX combination at 6.25 µg/ml. For both B. subtilis and M. luteus, MIC were recorded by BD-H+AMX at 1.56 µg/ml, MBC were observed at 3.13 and 1.56 µg/ml respectively, whereas for BD-H+PEN, it was found at 3.13 µg/ml and 0.78 µg/ml respectively, MBC obtained at 0.78 µg/ml. GER-H produced MIC at 0.78 µg/ml and MBC at 1.56 µg/ml for both combinations against B. subtilis. In case of M. luteus, GER-H exhibited bactericidal effect at 3.13 µg/ml for AMX combination, bacteriostatic effect for PEN and AMX combinations at 1.56 and 0.78 µg/ml respectively. Bacteriostatic effects were also exerted by AUS-H in combination with PEN and AMX at 1.56 and 0.78 µg/ml against B. subtilis, 3.13 and 1.56 µg/ml against M. luteus. MBC for AUS-H+AMX was observed at 1.56 µg/ml against B. subtilis. All three honeys showed neither bacteriostatic nor bactericidal effect against the bacterial strains when applied alone.
Discussion
Antimicrobial activities vary depending on the constituents which are subjected differ based on geo-locations. In this study, mixed honeys of different sources were screened by two different methods. Initial screening with disc diffusion assay revealed that the tested honey sample exhibited antibacterial activity against all the test bacteria. Microdilution assay explored the extent of activity in percentage and in addition drew a line between resistance and sensitivity. Further subculture differentiated between bactericidal effects from bacteriostatic effects.
Antibacterial susceptibility testing revealed that all the test bacteria exhibited high level of resistance to the standard antibiotics; similar observations were previously reported (15, 22). Penicillin, one of the most commonly prescribed antibiotics, contains a beta-lactam ring that works by inhibiting the transpeptidase enzyme, which are responsible for peptidoglycan cross-linking in both gram-positive and gram-negative bacteria (23). As a result, the cell becomes susceptible to lysis and can no longer withstand the osmotic stress (24). Resistance to β-lactam antibiotics continues to be a growing challenge, because of its widespread therapeutic dependence and misuse. Target modification of peptidoglycan-binding-proteins is the primary β-lactam-resistance mechanism in most gram-positive bacteria (25).
The third-generation penicillin, amoxicillin, has higher stability to penicillinase and thus exhibited better efficacy against a broader spectrum of bacteria that includes Escherichia coli, Haemophilus influenzae, Shigella spp and Salmonella spp. (24). However, it too is susceptible to degradation by beta-lactamase. Commercially available combinations of amoxicillin and clavulanic acid (beta-lactamase inhibitor) are known to be effective against a number of bacterial infections. The beta-lactamase inhibitors act by irreversibly binding to the beta-lactamase enzyme, thus preventing the cleavage of beta-lactam ring. Although these drugs do not possess inherent bactericidal activity, but they broaden amoxicillin's spectrum to bacteria that produce beta-lactamase enzyme (26).
Honey has re-emerged in modern medicine due to its potent antibacterial activity. It has been proposed that low pH, high osmolality, viscous properties and the enzymatic production of hydrogen peroxide (H2O2) are the major components that are responsible for the antibacterial activity of honey (27). Hydrogen peroxide is the most important antibacterial factor in natural honey, which is generated by the enzyme glucose oxidase (GOX) in diluted honey. This supported the reason why in most cases greater efficacies were obtained in diluted concentrations. GOX converts glucose to hydrogen peroxide and gluconic acid, under aerobic conditions (28). The concentration of hydrogen peroxide is quite low so it does not kill bacteria, however it does interact with bacterial cell proliferative signals even at diluted concentrations, therefore preventing bacterial growth to an extent (6). Similar results were observed with honeys and their combinations as shown in Figure 2 (a-d). Def-1, an antibacterial peptide, is also known to be effective against Gram-positive bacteria. Def-1 is present in variable quantities in honey, which is why the antibacterial activity differ among honey samples (6). Moreover, the antibacterial activity of honey is attributed to the presence of phytochemical constituents like thymoquinone, melanin, methylglyoxal (MGO) and its precursor dihydroxyacetone (DHA) apart from its nutritional elements- polyphenol, vitamin C, flavonoids and certain heavy metals. This is why apiarists are now trying to produce medical-grade honeys for wound care clinicians (29,30).
Osmosis, which occurs due to high sugar content, is also an important factor contributing to the antibacterial activity of honey samples. Undiluted honey has a high sugar content that exerts osmotic pressure against bacterial cells, resulting in transport of water out of the cells via osmosis. As a result, cells become dehydrated and lose their ability to multiply in hypertonic sugar solution (31). This explains why treatment with GER-H+AMX showed greater efficacy against S. pyogenes at less diluted concentration (6.25 µg/ml), in comparison to the other combinations. Apart from the low water activity, honey is mildly acidic (pH-3.9 to 4.8) and this alone is inhibitory to the growth of many pathogenic bacteria (32). More than 80% of soluble solids contributes to the dissolved sugar content and high amount of organic and amino acids is responsible for its low pH, therefore yielding antimicrobial properties (16).
Natural products such as honey have always played a pivotal role in the treatment of human diseases (33). Several studies have investigated the antibacterial activity honey on different bacterial strains. Honey, from different botanical and geographical origins, varies in their antibacterial potency. From the study it was quite evident that different microorganisms have different susceptibilities to different concentrations of honey subjected to different geographical sources. Further investigation by GC-MS and IR analysis is needed to determine the bioactive compounds responsible for the antimicrobial activity and the mechanism of action of honey on the pathogenic bacterial cells could be established using transmission electron microscopy (TEM) (34).
Conclusion
The rapid onset of bacterial resistance to most antibiotics has become a global public health concern as it not only diminishes the effectiveness of drugs but also creates a necessity for a constant supply of novel antibiotics for the treatment of bacterial infections. This study demonstrated that honeys derived from different geographical origins exhibited noteworthy antibacterial activity against gram-positive pathogens. Combinations of honey and amoxiclav showed greater potential in inhibiting bacterial growth than either of them alone, thus indicating a synergistic effect. Therefore, use of honey with conventional antibiotics could be a promising approach to enhance its efficacy and promote its antibacterial properties.
Abbreviations
PEN: Penicillin; AMX: Amoxicillin-Clavulanic Acid (Amoxiclav); BD-H: Honey sourced from Bangladesh; GER-H: Honey sourced from Germany; AUS-H: Honey sourced from Australia; MHA: Muller Hinton Agar; NB: Nutrient Broth; ATCC: American Type Culture Collection; MIC: Minimum Inhibitory Concentration; MBC: Minimum Bactericidal Concentration; GOX: Glucose Oxidase; Def-1: Bee Defensin-1; MGO: Methylglyoxal; DHA: Dihydroxyacetone; OD: Optical Density; IR: Infra-Red; GC-MS: Gas Chromatography–Mass Spectrometry; TEM: transmission electron microscopy.
Ethics Approval and Consent to Participate
The work was exempt for ethical approval. All experiments were performed in accordance with Clinical and Laboratory Standards Institute (CLSI)’s standards for microbial testing - M02: Performance Standards for Antimicrobial Disk Susceptibility Tests, 13th Edition; M100: Performance Standards for Antimicrobial Susceptibility Testing, 31st Edition; M26: Methods for Determining Bactericidal Activity of Antimicrobial Agents, 1st Edition; M29: Protection of Laboratory Workers From Occupationally Acquired Infections, 4th Edition, adopted in the Institutional Standard Microbial Testing Procedures (Ref. no. IPSDRLAB/ ISMTP/01/19). The experimental design was authorized by the Institutional Ethical Committee Clearance (Ref. No. IPSDRLAB/IECC/20/ 19) of the Institute for Pharmaceutical Skill Development and Research, Bangladesh (Project approved on 17/02/2019).
Acknowledgments
The present study was supported and carried out in the Pharmacology lab of Institute for Pharmaceutical Skill Development and Research, Bangladesh. Authors are grateful to the institution for providing such opportunity to contribute to health science. Authors are also grateful to the Center for Medical Biotechnology under MIS, DGHS, for providing the bacterial strains used in this study.
Authors’ Contributions
This work was carried out in collaboration between all authors. Authors MNI and MMB designed, coordinated and supervised the project. KN and FA performed the experiments. FA prepared the manuscript and together with MNI participated in interpretation of data to reach a scientific discussion. AH participated in the physicochemical assessment and microdilution techniques. MMB prepared the graphical presentations and critically revised the manuscript. All authors read and approved the final manuscript.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors. The study was carried out with individual funding of all authors.
Availability of Data and Materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Consent for Publication
Not applicable.
Competing Interests
All authors agreed on the article before submission and had no conflict of interests.
References
- Mandal MD, Mandal S. Honey: Its medicinal property and antibacterial activity. Asian Pac J Trop Biomed. 2011; 1(2):154–60. DOI PMC Google Scholar Link
- Das R, Romi W, Das R, Sharma HK, Thakur D. Antimicrobial potentiality of actinobacteria isolated from two microbiologically unexplored forest ecosystems of Northeast India. BMC Microbiol. 2018; 18(1):71. DOI PubMed Google Scholar Link
- Majoumouo MS, Sibuyi NRS, Tincho MB, Mbekou M, Boyom FF, Meyer M. Enhanced anti-bacterial activity of biogenic silver nanoparticles synthesized from Terminalia mantaly extracts. Int J Nanomedicine. 2019; 14:9031–46. DOI PMC Google Scholar Link
- Tadesse BT, Ashley EA, Ongarello S, Havumaki J, Wijegoonewardena M, González IJ, et al. Antimicrobial resistance in Africa: A systematic review. BMC Infect Dis. 2017; 17(1):616. DOI PubMed Google Scholar Link
- Nishio EK, Ribeiro JM, Oliveira AG, Andrade CGTJ, Proni EA, Kobayashi RKT, et al. Antibacterial synergic effect of honey from two stingless bees: Scaptotrigona bipunctata Lepeletier, 1836, and S. postica Latreille, 1807. Sci Rep. 2016; 6. DOI PubMed Google Scholar Link
- Bucekova M, Jardekova L, Juricova V, Bugarova V, Di Marco G, Gismondi A, et al. Antibacterial activity of different blossom honeys: New findings. Molecules. 2019;24(8). DOI PubMed Google Scholar Link
- 7. Stagos D, Soulitsiotis N, Tsadila C, Papaeconomou S, Arvanitis C, Ntontos A, et al. Antibacterial and antioxidant activity of different types of honey derived from Mount Olympus in Greece. Int J Mol Med. 2018;42(2):726–34. DOI PubMed PMC Google Scholar Link
- Nolan VC, Harrison J, Cox JAG. Dissecting the Antimicrobial Composition of Honey. Antibiotics. 2019;8(4):251. DOI PubMed PMC Google Scholar Link
- Fyfe, L.; Okoro, P.; Paterson, E.; Coyle, S.; Mcdougall, G.J. Compositional analysis of Scottish honeys with antimicrobial activity against antibiotic-resistant bacteria reveals novel antimicrobial components. LWT Food Sci. Technol. 2017, 79, 52–59. DOI Google Scholar Link
- Laallam, H.; Boughediri, L.; Bissati, S.; Menasria, T.; Mouzaoui, M.S.; Hadjadj, S.; Hammoudi, R.; Chenchouni, H. Modeling the synergistic antibacterial effects of honey characteristics of different botanical origins from the Sahara Desert of Algeria. Front. Microbiol. 2015, 6, 1–12. DOI PubMed Google Scholar Link
- Alvarez-suarez, J.M.; Tulipani, S.; Díaz, D.; Estevez, Y.; Romandini, S.; Giampieri, F.; Damiani, E.; Astolfi, P.; Bompadre, S.; Battino, M. Antioxidant and antimicrobial capacity of several monofloral Cuban honeys and their correlation with color, polyphenol content and other chemical compounds. Food Chem. Toxicol. 2010, 48, 2490–2499. DOI PubMed Google Scholar Link
- Miguel MG, Antunes MD, Faleiro ML. Honey as a complementary medicine. Integrative Medicine Insights. 2017;12:1–15. DOI PMC Google Scholar Link
- Al-Waili N, Al-Ghamdi A, Ansari MJ, Al-Attal Y, Salom K. Synergistic effects of honey and propolis toward drug multi-resistant Staphylococcus Aureus, Escherichia coli and Candida Albicans isolates in single and polymicrobial cultures. Int J Med Sci. 2012;9(9):793–800. DOI PubMed Google Scholar Link
- Khan M, Islam Z, Chowdhury AS, Yousuf SA, Amin MR, Rayhan MA. Wild Honey Facilitates Antibacterial Efficacy of Penicillin and Amoxicillin-Clavulanic Acid. Journal of Apitherapy. 2020;7(2):22–30 DOI Google Scholar Link
- Hossain R, Rahman MS, Rayhan MA, Nawrin K, Billah MM, Habib MR. Antibacterial efficacy of black seed honey in combination with penicillin and amoxiclav against gram-positive bacteria. Int J Sci Rep 2020;6(2):61-6. DOI Google Scholar Link
- Vabna NJ. Lychee Honey Accelerates Antibacterial Efficacy of Penicillin and Amoxiclav Against Gram-Positive Bacteria in Libya. Am J Biomed Sci Res. 2019;6(3):226–31. DOI Google Scholar Link
- Rayhan MA, Vabna NJ, Ahmed F, Hossin A, Nawrin K, Billah MM. Jujube (Ziziphus jujube) honey treats stress induced anxiety behavior in mice. Pharmacotherapy and Pharmascience Discovery. 2021;1(1):1-9. Google Scholar Link
- Mostafa AA, Al-Askar AA, Almaary KS, Dawoud TM, Sholkamy EN, Bakri MM. Antimicrobial activity of some plant extracts against bacterial strains caus¬ing food poisoning diseases. Saudi J Biol Sci 2018; 25(2):361–6; doi: 10.1016/j.sjbs.2017.02.004. DOI PubMed PMC Google Scholar Link
- Patton T, Barett J, Brennan J, Moran N. Use of a spectrophotometric bioassay for determination of microbial sensitivity to manuka honey. J Microbiol Methods 2005; 64:84–95. DOI PubMed Google Scholar Link
- Wiegand I, Hilpert K, Hancock REW. Agar and broth dilution methods to determine the minimal inhibitory concentration (MIC) of antimicrobial substances. Nat Protoc. 2008;3(2):163-175. DOI PubMed Google Scholar Link
- M26AE: Bactericidal Activity of Antimicrobial Agents. Clinical & Laboratory Standards Institute. Accessed June 15, 2021. Link
- Bouacha M, Ayed H, Grara N. Honey bee as alternative medicine to treat eleven multidrug resistant bacteria causing urinary tract infection during pregnancy. Sci Pharm. 2018;86(2). DOI PubMed PMC Google Scholar Link
- Bush K, Bradford PA. β-lactams and β-lactamase inhibitors: An overview. Cold Spring Harb Perspect Med. 2016;6(8). DOI PMC Google Scholar Link
- Lobanovska M, Pilla G. Penicillin’s discovery and antibiotic resistance: Lessons for the future? Yale J Biol Med. 2017;90(1):135–45. PubMed PMC Google Scholar Link
- Fisher JF, Mobashery S. β-Lactam resistance mechanisms: Gram-positive bacteria and mycobacterium tuberculosis. Cold Spring Harb Perspect Med. 2016;6(5). DOI PubMed PMC Google Scholar Link
- Kwon YH. Amoxicillin. In: Helicobacter pylori [Internet]. Springer Singapore; 2016 [cited 2020 Oct 29]. p. 387–96. DOI PMC Google Scholar Link
- Irish J, Blair S, Carter DA. The antibacterial activity of honey derived from Australian flora. PLoS One. 2011;6(3):18229. DOI PubMed Google Scholar Link
- Tan HT, Rahman RA, Gan SH, Halim AS, Hassan SA, Sulaiman SA, et al. The antibacterial properties of Malaysian tualang honey against wound and enteric microorganisms in comparison to manuka honey. BMC Complement Altern Med. 2009;9:34. DOI PubMed PMC Google Scholar Link
- Forouzanfar F, Fazly Bazzaz BS, Hosseinzadeh H. Black cumin (Nigella sativa) and its constituent (thymoquinone): A review on antimicrobial effects. Iran J Basic Med Sci. 2014; 17(12):929–38. PubMed PMC Google Scholar Link
- Cokcetin NN, Pappalardo M, Campbell LT, Brooks P, Carter DA, Blair SE, et al. The antibacterial activity of Australian Leptospermum honey correlates with methylglyoxal levels. PLoS One. 2016;11(12). DOI PubMed PMC Google Scholar Link
- Albaridi NA. Antibacterial Potency of Honey. Int J Microbiol. 2019;2019. DOI PubMed PMC Google Scholar Link
- Alzahrani HA, Alsabehi R, Boukraâ L, Abdellah F, Bellik Y, Bakhotmah BA. Antibacterial and antioxidant potency of floral honeys from different botanical and geographical origins. Molecules. 2012;17(9):10540–9. DOI PubMed PMC Google Scholar Link
- Butler MS, Buss AD. Natural products - The future scaffolds for novel antibiotics? Biochem Pharmacol. 2006 Mar 30;71(7):919–29. DOI PubMed Google Scholar Link
- Abdel-Shafi S, Al-Mohammadi AR, Hamdi S, Moustafa AH, Enan G. Biological characterization and inhibition of streptococcus pyogenes ZUH1 causing chronic cystitis by crocus sativus methanol extract, bee honey alone or in combination with antibiotics: An in vitro study. Molecules. 2019;24(16). DOI PubMed PMC Google Scholar Link
Update History
| Revision Number | Date | Details Of Changes |
|---|---|---|
| 01 | 2021-06-16 | Original Article; published at its accepted version (Reference Number: PPD/MIN/2162A) |
 Pharmacotherapy & Pharmascience Discovery
Pharmacotherapy & Pharmascience Discovery
